VOORBLAD; IN TE VULLEN DOOR AANVRAGER
Datum 10/02/2023
Indicatieaanvraag/studie: Docetaxel en trastuzumab bij HER2 positief speekselkliercarcinoom (SDC)
VOORBLAD; IN TE VULLEN DOOR CieOOM
Datum 18/04/23
Beoordeling door CieOOM mogelijk: ja
Beoordeling voorleggen aan CieBOM: ja/nee; lijkt geen toegevoegde waarde te hebben als cieOOM kan beoordelen cf NRS
Is er volgens CieOOM een gerandomiseerde studie mogelijk: nee
Waar hoort beoordeling thuis:
- Zeldzame indicatie/NRS
Huidige standaard: ESMO guidelines 2022;
“Agents targeting androgen receptors and/or HER2 are promising and are the best studied therapies in patients with salivary duct carcinoma.”
Huidige indicatietekst (Farmacotherapeutisch Kompas)
Docetaxel; diverse indicaties voor mammacarcinoom, NSCLC, prostaatcarcinoom, adenocarcinoom van de maag, hoofd/halscarcinoom
Trastuzumab; vroeg en gemetastaseerd HER2-positief mammacarcinoom, gemetastaseerd HER2-positief adenocarcinoom van de maag of de gastro-oesofageale overgang (níét de Herceptin s.c. injectie)
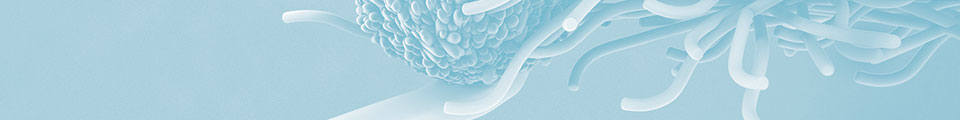
Einddatum patent: voor beide middelen reeds verlopen
Zeldzame indicatie/NRS
Tumortype en behandellijn
HER2 positief SDC
Medicament/middelen
Trastuzumab icm docetaxel
Indicatie (EMA) en huidige indicatietekst (op farmatec)
Herceptin is used to treat the following types of cancer:
- early breast cancer (when the cancer has spread within the breast or to the glands under the arm but not to other parts of the body) after surgery, chemotherapy (medicines to treat cancer), and radiotherapy (treatment with radiation) if applicable. It can also be used earlier in treatment, in combination with chemotherapy. For tumours that are locally advanced (including those that are inflammatory) or more than 2 cm wide, Herceptin is used before surgery in combination with chemotherapy and then again after surgery on its own;
- metastatic breast cancer (cancer that has spread to other parts of the body). It is used on its own in patients in whom previous treatments have failed. It is also used in combination with other anticancer medicines: with paclitaxel or docetaxel, or with an aromatase inhibitor;
When used as an infusion into a vein, Herceptin can also be used for:
- metastatic gastric (stomach) cancer, in combination with cisplatin and either capecitabine or 5-fluorouracil (other anticancer medicines).
Herceptin can only be used when the cancer has been shown to ‘overexpress HER2’: this means that the cancer produces a protein called HER2 in large quantities on the surface of the tumour cells, which makes the tumour cells grow more quickly. HER2 is overexpressed in about a quarter of breast cancers and a fifth of gastric cancers.
Korte bespreking van de studie(s)
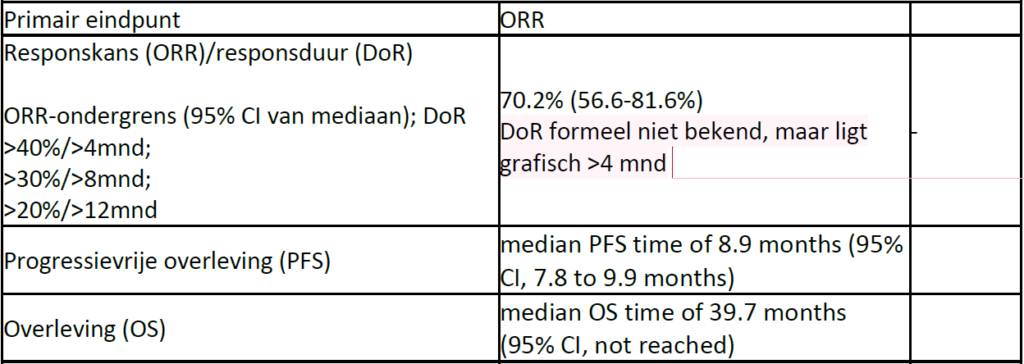
This was a single-center, single-arm, open-label, phase II study in Japan. The patients received trastuzumab at a loading dose of 8 mg/kg, followed by 6 mg/kg every 3 weeks. Docetaxel 70 mg/m 2 was administrated every 3 weeks. The primary end point was the overall response rate.
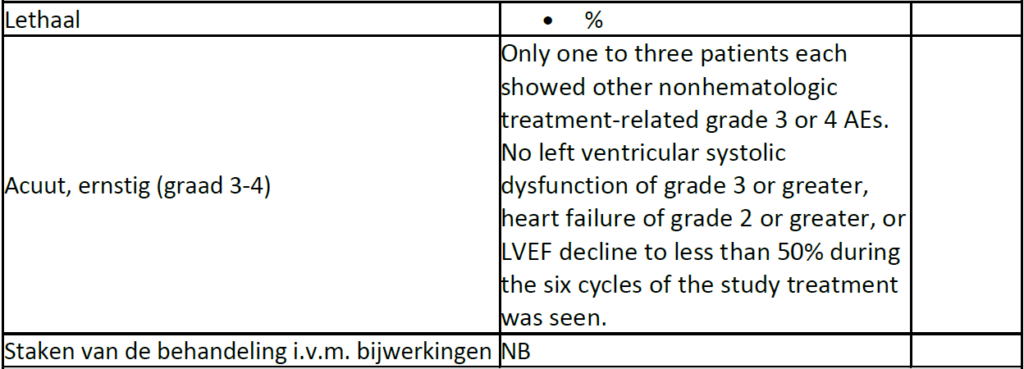
Fifty-seven eligible patients with SDC were enrolled. The overall response rate was 70.2% (95% CI, 56.6% to 81.6%), and the clinical benefit rate was 84.2% (95% CI, 72.1% to 92.5%). Median progression-free and overall survival times were 8.9 months (95% CI, 7.8 to 9.9 months) and 39.7 months (95% CI, not reached), respectively. The most frequent adverse event was anemia (52 patients [91%]), followed by a decreased WBC count (51 patients [89%]) and neutropenia (50 patients [88%]). The most frequently observed grade 4 adverse event was a decreased neutrophil count (34 patients [60%]). Grade 3 febrile neutropenia was reported in eight patients (14%). No grade 2 or greater adverse events of heart failure or left ventricular ejection fraction decline to less than 50% occurred.
Past studieopzet bij de zeldzaamheid van de indicatie?
ja
Effectiviteit
Bijwerkingen (totaal/gerelateerd aan de behandeling)
Kwaliteit van leven
Kwaliteit van leven analyse: nv
Beperkingen van de studie
Kleine singlecenter fase II studie uit Japan
Alleen HER 2 positieve SDC; betreft 30-40% van alle patiënten.
AR positiviteit komt vaker voor (daar doet UMCRadboud nu studies mee)
Veel case series in de literatuur aangaande HER2 positieve SDC; meestal gerichte anti HER2 behandeling(en) in diverse samenstellingen, lang niet altijd icm chemotherapie/docetaxel
Is het te overwegen alleen anti HER 2 behandeling in te zetten?
Referentie(s)
Takahashi H, Tada Y, Saotome T et al. Phase II Trial of Trastuzumab and Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2–Positive Salivary Duct Carcinoma. J Clin Oncol. 2019 Jan 10;37(2):125-134
Discussie / advies / wanneer herbeoordelen
Akkoord voor off-label vergoeding van deze combinatie
Datum besproken vergadering
18-04-2023